About PULIH
Low Back Pain Monitoring Device
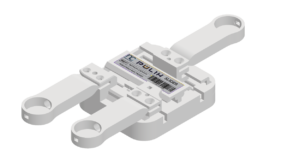
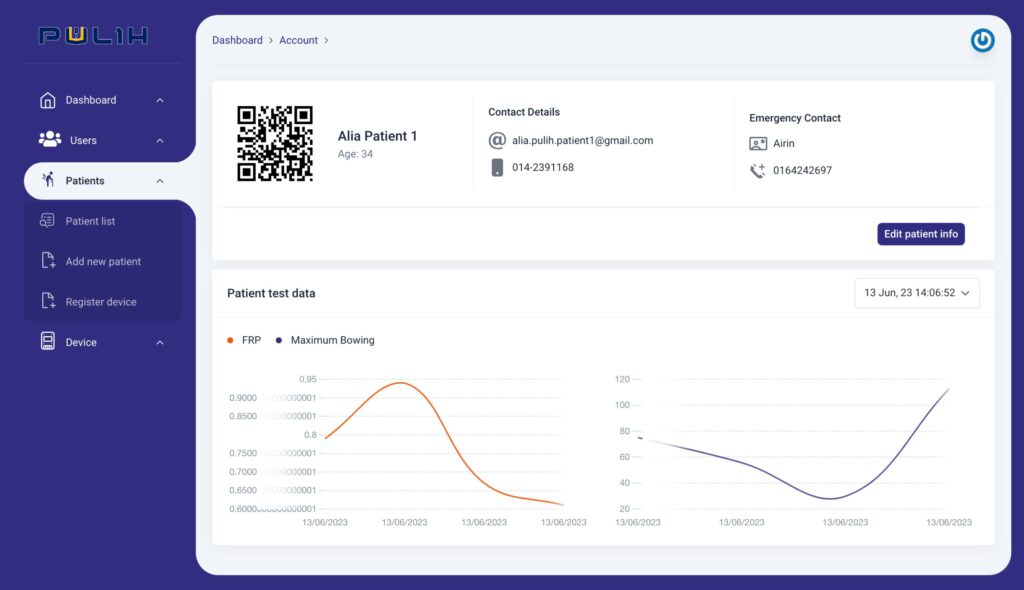
The PULIH Mobile EMG Lower Back Pain Monitor is a battery-powered device (NCPULIH-22BT01MY) intended to automate the diagnosis of Lower Back Pain by utilising specialised algorithms. This makes detection reliable, quantifiable, and straightforward. The device features remote monitoring, allowing data collecting to occur with and without close clinical supervision. Normal personal user can receive free training to identify their electrode placement locations.
PULIH is equipped with Bluetooth connection that is always available when PULIH is switched on and can be made through PULIH mobile application during setup. The device integrated with sensors that is essential to calculate the Lower Back Pain algorithm. The device contains replaceable and rechargeable lithium-ion battery. The battery status can be monitored via the LED indicator. Battery replacement can only be conducted by certified personnel, as per guarantees regulation. Our Eco-friendly product design policy make it so that the device will automatically shut down after 15 minutes idling.
The device main housing function together with the slider housing that connect to the electrode. The slider can be removed and be replaced with another, according to the user’s body type and size. The placement is adjustable assuming the position of the electrode. To replace any part, the slider leg and battery require to be done by authorise personnel or else the warranty of the device will be void.
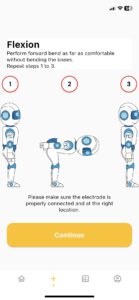
How It Works

Specifications & Manual
| GENERAL SPECIFICATION | |
| DIMENSION (MAIN PULIH DEVICE) | (H)90MM X (W)100MM X (L)40MM |
| WEIGHT | 120g |
| ENCLOSURE MATERIAL | POLYURETHENE |
| ELECTRICAL PROPERTIES | |
| CHARGING VOLTAGE | 5.0v |
| CHARGING CURRENT | 1.0A |
| WORKING VOLTAGE | 3.3v |
| BATTERY TECHNOLOGY | LITHIUM – ION (18650) |
| WIRELESS TECHNOLOGY | BLUETOOTH 5 LOW ENERGY |
| ENVIRONMENTAL CONDITION | |
| OPERATING CONDICTION | 15 ℃ TO 40 ℃ |
| 10% – 90% RELATIVE HUMIDITY (NONCONDENSING) | |
| STORAGE CONDITION | 15 ℃ TO 40 ℃ |
| 10% – 90% RELATIVE HUMIDITY (NONCONDENSING) | |
| EMG SPECIFICATION | |
| CHANNEL | SINGLE DIFFERENTIAL CHANNEL |
| SAMPLING RATE | 5,000 Hz |
| BANDPASS FREQUENCY | 5 – 1000 Hz |
| FEATURES | |
| SINGLE BUTTON CONTROL | ON AND OFF MEMBRAN SWITCH |
| LED INDICATION | INDICATES BETWEEN CONNECTION AND MEASUREMENT STATE |
| TYPE-C USB | CHARGING PORT |
| MOBILE APPLICATION | USER OPERATION AND MEASUREMENT |
| DOCTOR DASHBOARD | ADMINSTRATION AND REMOTE PATIENT PROGRESS MONITOR |
| ADJUSTABLE ELECTRODE SLIDER | ERROR PROOF MAGNETIC CONNECTOR FOR ELECTRODE. ELECTRODE WIDTH AND DISTANCE ADJUSTABLE MECHANISM |
| SNAP ADAPTOR | SINGLE USE BIOPOTENTIAL ELECTRODE SNAP METHOD |
| SAFETY STANDARDS | IEC 60601-1 (IN PROGRESS) |
| IEC 60601-1 -2 EMC (IN PROGRESS) | |
| EXPECTED SERVICE LIFETIME | |
| BATTERY CHARGING COUNTS | ~500 CYCLES (NCR18650B) |
| SLIDER – ELECTRODE LEADS | ~2000 COUNTS OF SNAP IN & OUT |
| PULIH MAIN HARDWARE | 4 YEARS |
Download Now:

Apple App Store

Google Play Store
Mobile Apps Preview
Painless
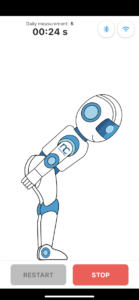
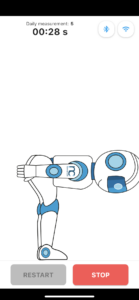
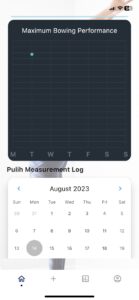
Non-invasive approach to measure lower back pain
Easy
Easy to use with device and mobile applications
Near
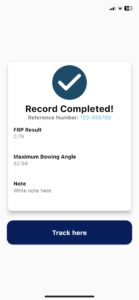
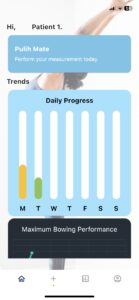
Recovery process at the fingertips of your physician